The spikes are called absorption bands in an IR spectrum. A molecule have a variety of covalent bonds, and each bond have different vibration modes, so the IR spectrum of a compound usually show multiple absorption bands. The horizontal axis indicates the position of an absorption band. But instead of using frequency to show the absorbed
Processes | November 2020 – Browse Articles
Feb 8, 2023at what approximate positions might this compound show ir absorptions? consult the table given above; there must be an exact match of groups with the absorptions. there are occasional overlaps between absorptions in the table, be sure that the group is actually present in the molecule. esters and alcohols both show similar c-o stretching vibrations. enter your choices as an alphabetized string

Source Image: scribd.com
Download Image
At what approximate positions might this compound show IR absorptions? Consult the table given above; there must be an exact match of groups with the absorptions. There are occasional overlaps between absorptions in the table, be sure that the group is actually present in the molecule.
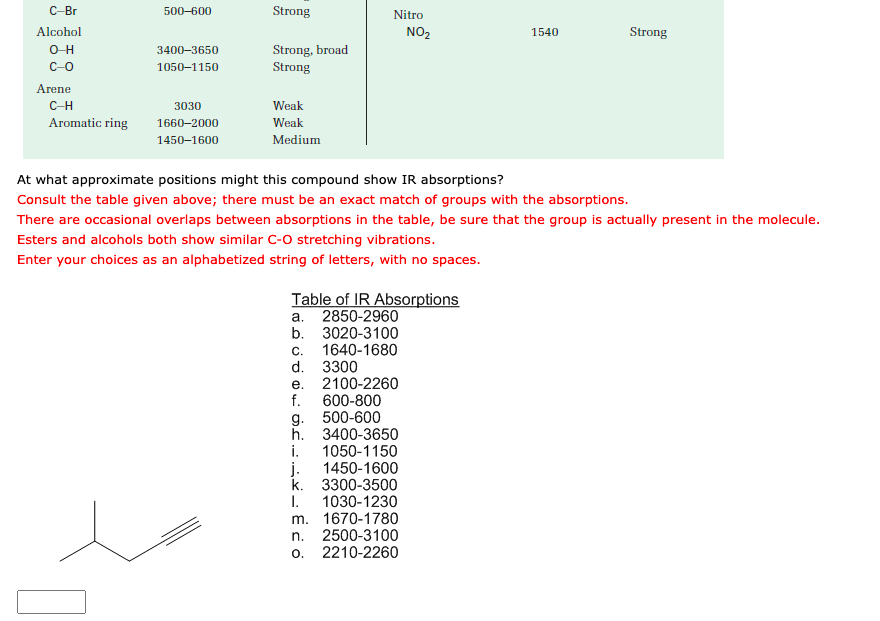
Source Image: chegg.com
Download Image
elites1a4s1z4.jpg Describe the prominent IR absorptions and mass spectral peaks you would expect for the following compound: What steps transform the graph of y=x^2 y =x2 to the graph of the function below. Find step-by-step Chemistry solutions and your answer to the following textbook question: At what approximate positions might the following compounds show IR

Source Image: chemistry-europe.onlinelibrary.wiley.com
Download Image
At What Approximate Positions Might This Compound Show Ir Absorptions
Describe the prominent IR absorptions and mass spectral peaks you would expect for the following compound: What steps transform the graph of y=x^2 y =x2 to the graph of the function below. Find step-by-step Chemistry solutions and your answer to the following textbook question: At what approximate positions might the following compounds show IR Oct 7, 2023Step 1/5 1. CH:CHzCOCH3: This compound contains a C=C bond and a C=O bond. The C=C bond typically absorbs in the range of 1600-1680 cm-1, while the C=O bond absorbs in the range of 1700-1750 cm-1. Therefore, we can expect absorptions around 1650 cm-1 for the C=C bond and around 1730 cm-1 for the C=O bond. Step 2/5 2.
Perturbation of the NH2 pKa Value of Adenine in Platinum(II) Complexes: Distinct Stereochemical Internucleobase Effects – Garijo Añorbe – 2004 – Chemistry – A European Journal – Wiley Online Library
Sep 29, 2023Problem 12-32 Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexene. Identify them, and explain your answer. (a) (b) Problem 12-33 At what approximate positions might the following compounds show IR absorptions? Solved At what approximate positions might this compound | Chegg.com
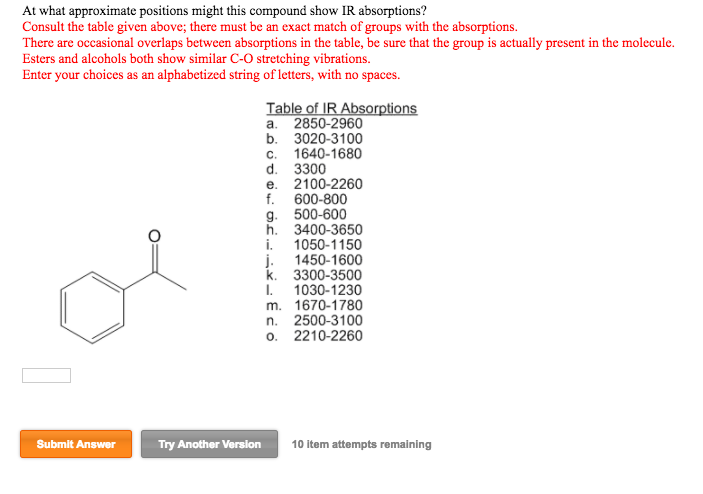
Source Image: chegg.com
Download Image
IR: nitro groups Sep 29, 2023Problem 12-32 Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexene. Identify them, and explain your answer. (a) (b) Problem 12-33 At what approximate positions might the following compounds show IR absorptions?

Source Image: orgchemboulder.com
Download Image
Processes | November 2020 – Browse Articles The spikes are called absorption bands in an IR spectrum. A molecule have a variety of covalent bonds, and each bond have different vibration modes, so the IR spectrum of a compound usually show multiple absorption bands. The horizontal axis indicates the position of an absorption band. But instead of using frequency to show the absorbed

Source Image: mdpi.com
Download Image
elites1a4s1z4.jpg At what approximate positions might this compound show IR absorptions? Consult the table given above; there must be an exact match of groups with the absorptions. There are occasional overlaps between absorptions in the table, be sure that the group is actually present in the molecule.

Source Image: sec.gov
Download Image
⏩SOLVED:At what approximate positions might the following compounds… | Numerade Expert solutions Question Where might the following compound have IR absorptions? Solution Verified Create an account to view solutions CHEMISTRY \mathrm C_ 6 \mathrm H_ 10 \mathrm O_ 2 H cm CHEMISTRY
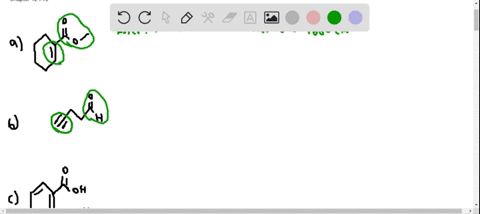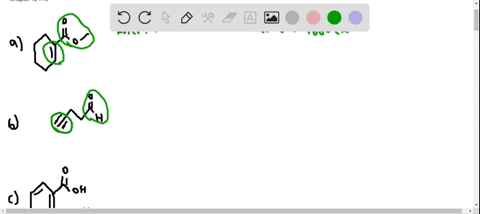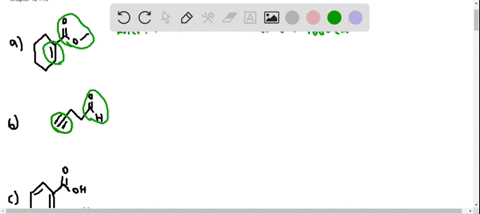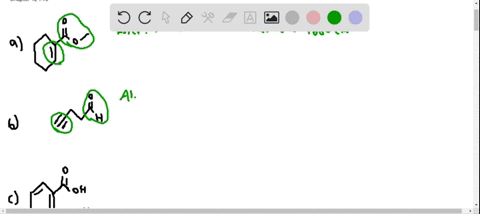
Source Image: numerade.com
Download Image
Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response | Pharmacological Reviews Describe the prominent IR absorptions and mass spectral peaks you would expect for the following compound: What steps transform the graph of y=x^2 y =x2 to the graph of the function below. Find step-by-step Chemistry solutions and your answer to the following textbook question: At what approximate positions might the following compounds show IR

Source Image: pharmrev.aspetjournals.org
Download Image
Solved 3. Four infrared spectra are shown below. Assign each | Chegg.com Oct 7, 2023Step 1/5 1. CH:CHzCOCH3: This compound contains a C=C bond and a C=O bond. The C=C bond typically absorbs in the range of 1600-1680 cm-1, while the C=O bond absorbs in the range of 1700-1750 cm-1. Therefore, we can expect absorptions around 1650 cm-1 for the C=C bond and around 1730 cm-1 for the C=O bond. Step 2/5 2.
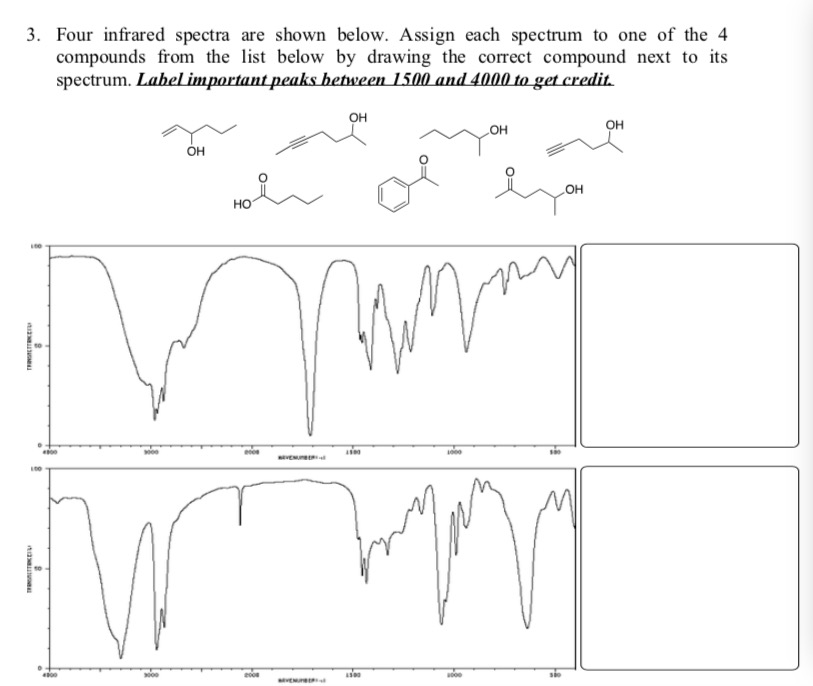
Source Image: chegg.com
Download Image
IR: nitro groups
Solved 3. Four infrared spectra are shown below. Assign each | Chegg.com Feb 8, 2023at what approximate positions might this compound show ir absorptions? consult the table given above; there must be an exact match of groups with the absorptions. there are occasional overlaps between absorptions in the table, be sure that the group is actually present in the molecule. esters and alcohols both show similar c-o stretching vibrations. enter your choices as an alphabetized string
elites1a4s1z4.jpg Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Response | Pharmacological Reviews Expert solutions Question Where might the following compound have IR absorptions? Solution Verified Create an account to view solutions CHEMISTRY \mathrm C_ 6 \mathrm H_ 10 \mathrm O_ 2 H cm CHEMISTRY