Figure 3.2.5 The Atomic Radius of the Elements. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. The increase in atomic size going down a column is also due to electron shielding, but the situation is more complex because the principal quantum number n is not constant.
electro and radii make up quiz
Aug 24, 2023In decreasing order of atomic radius, the elements rank as follows: Tin (Sn), Germanium (Ge), Silicon (Si), and Carbon (C). Atomic radius generally increases as you move down a group and decreases as you move across a period on the periodic table. Explanation:
Source Image: bartleby.com
Download Image
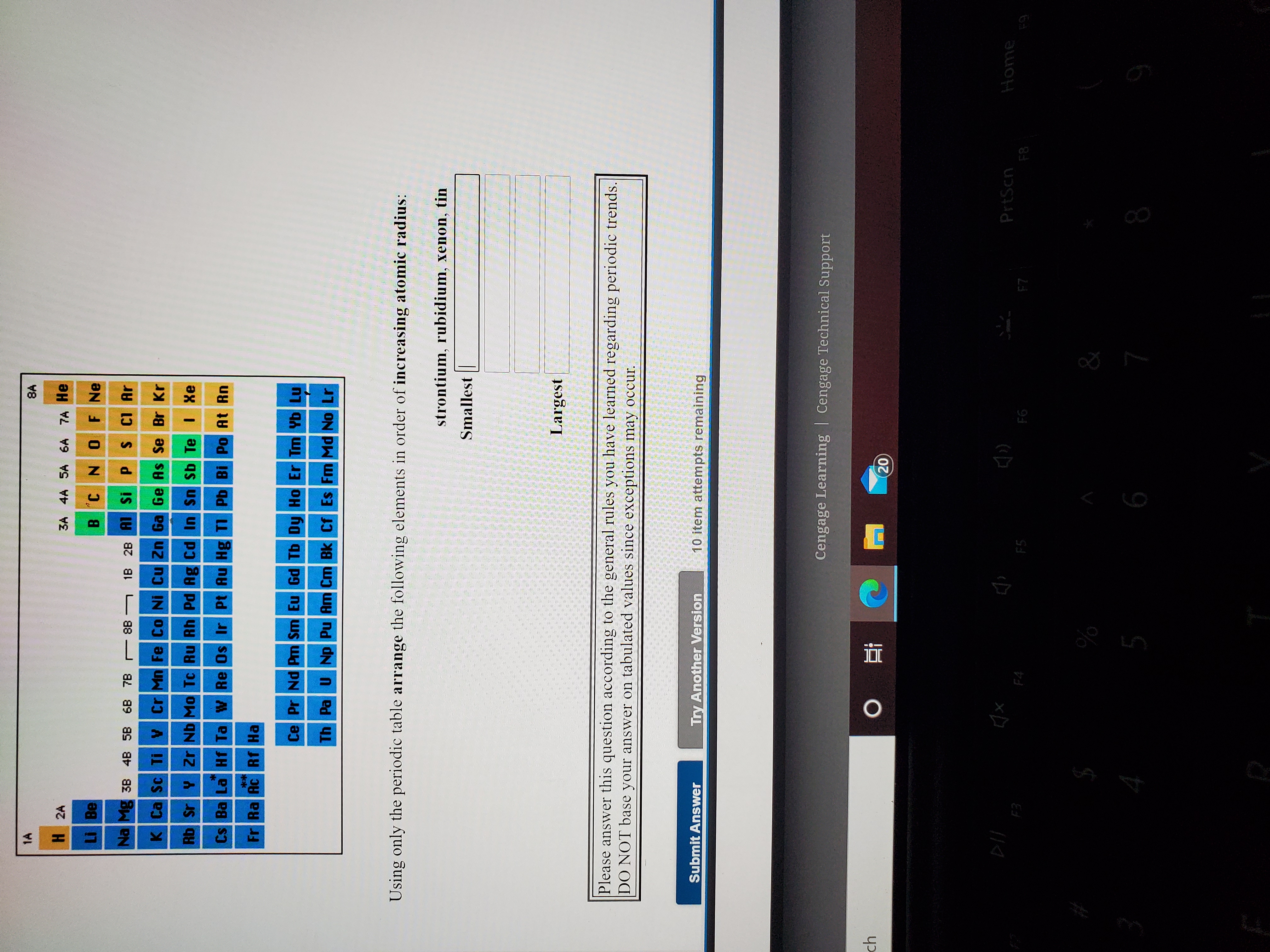
Arrange the following atoms according to decreasing effective nuclear charge experienced by their valence electrons: SS, Mg, Al, and Si. Click the card to flip 👆 least to greatest, Mg, Al, Si, S Click the card to flip 👆 1 / 70 Flashcards Learn Test Match Q-Chat Created by anderson10296 chapter 9= pink, chapter 8=blue, chapter 7= yellow

Source Image: homework.study.com
Download Image
Atomic Radius Trend | Periodic Table | ChemTalk Science Chemistry Chemistry questions and answers Part B Rank the following elements in order of decreasing atomic radius. Rank from largest to smallest radius. To rank items as equivalent, overlap them. View Available Hint (s) Reset Help 0000 Largest Smallest Na Mg AI SI Rank from largest to smallest radius.

Source Image: brainly.com
Download Image
Rank The Following Elements In Order Of Decreasing Atomic Radius
Science Chemistry Chemistry questions and answers Part B Rank the following elements in order of decreasing atomic radius. Rank from largest to smallest radius. To rank items as equivalent, overlap them. View Available Hint (s) Reset Help 0000 Largest Smallest Na Mg AI SI Rank from largest to smallest radius. Nov 16, 2023Hence, the order for the elements – Ar, Fr, Cl, Xe, and Y in decreasing atomic radius would be Fr > Xe > Ar > Cl > Y. To explain this, Francium (Fr) has the largest atomic radius because it is in the bottom of the periodic table, meaning it has the most energy levels or shells.
Rank the following elements in order of decreasing atomic radius (1 is biggest, 5 is smallest) Nitrogen – brainly.com
Rank the following elements in order of decreasing atomic radius. rank from largest to smallest radius. to rank items as equivalent, overlap them. SOLVED: 3- Arrange the following elements in order of decreasing atomic radius: Cs, Sb, S, Pb, Se 4- Write the electronic configurations for each of the following ions: a. O2- b. Br-

Source Image: numerade.com
Download Image
Which arrangement is in the correct order of radius size? a) Mn > Mn2+ > Cs b) Li+ > Li > Ra c) P < P3– < As3– d) Cr < Cr3+ < Ca e) Al3+ > Al > Si | Socratic Rank the following elements in order of decreasing atomic radius. rank from largest to smallest radius. to rank items as equivalent, overlap them.
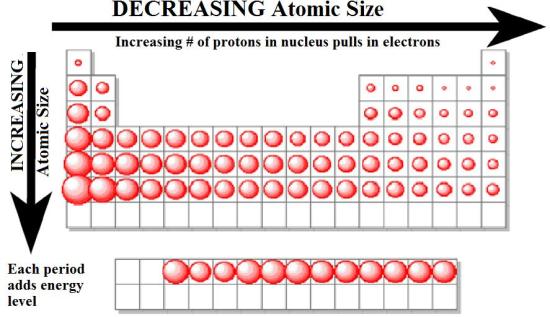 Download Image
Download Imageelectro and radii make up quiz Figure 3.2.5 The Atomic Radius of the Elements. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. The increase in atomic size going down a column is also due to electron shielding, but the situation is more complex because the principal quantum number n is not constant.
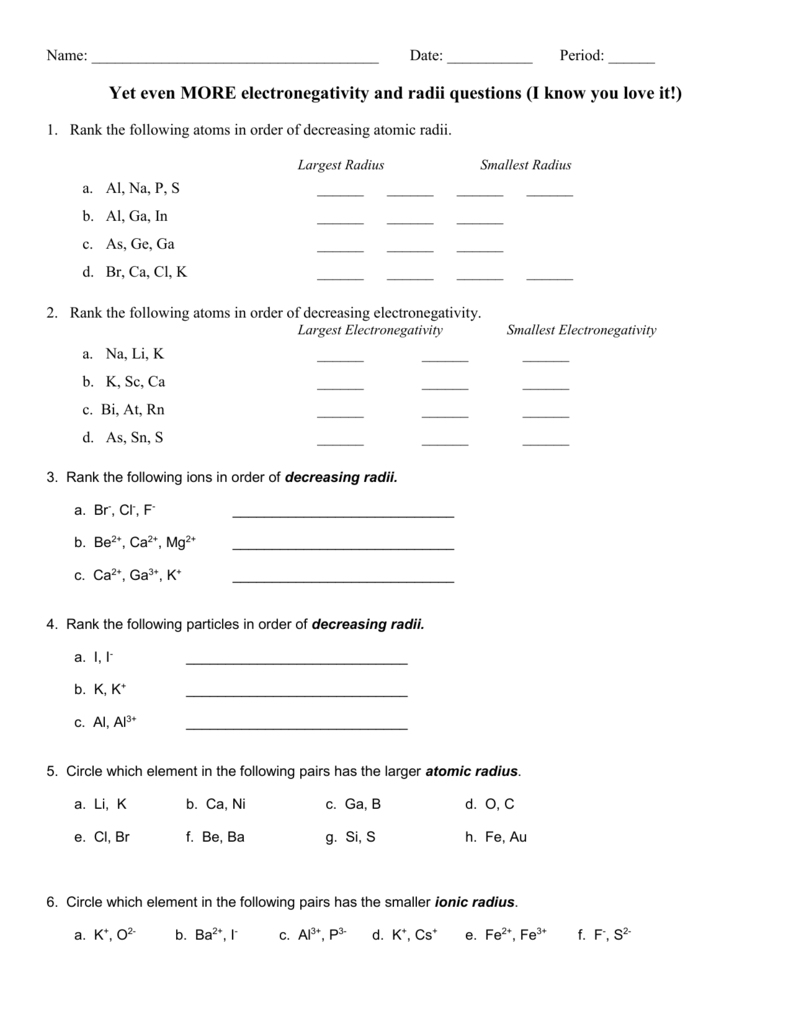
Source Image: studylib.net
Download Image
Atomic Radius Trend | Periodic Table | ChemTalk Arrange the following atoms according to decreasing effective nuclear charge experienced by their valence electrons: SS, Mg, Al, and Si. Click the card to flip 👆 least to greatest, Mg, Al, Si, S Click the card to flip 👆 1 / 70 Flashcards Learn Test Match Q-Chat Created by anderson10296 chapter 9= pink, chapter 8=blue, chapter 7= yellow

Source Image: chemistrytalk.org
Download Image
Rank the following elements in order of decreasing atomic radius…. | Channels for Pearson+ VIDEO ANSWER: It’s helpful to know the trend with a periodic table if you want to rank the elements in order of increasing atomic radius. The size of the nucleus is going to go down. We see a decrease in atomic radii.

Source Image: pearson.com
Download Image
Periodic Trends in Atomic Size | CK-12 Foundation Science Chemistry Chemistry questions and answers Part B Rank the following elements in order of decreasing atomic radius. Rank from largest to smallest radius. To rank items as equivalent, overlap them. View Available Hint (s) Reset Help 0000 Largest Smallest Na Mg AI SI Rank from largest to smallest radius.
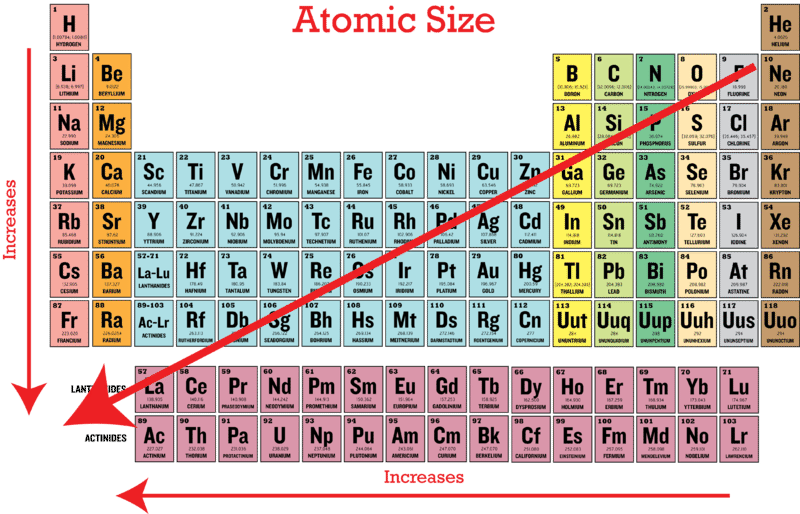
Source Image: ck12.org
Download Image
Periodic Trend: Atomic Radius Video Tutorial & Practice | Channels for Pearson+ Nov 16, 2023Hence, the order for the elements – Ar, Fr, Cl, Xe, and Y in decreasing atomic radius would be Fr > Xe > Ar > Cl > Y. To explain this, Francium (Fr) has the largest atomic radius because it is in the bottom of the periodic table, meaning it has the most energy levels or shells.
Source Image: pearson.com
Download Image
Which arrangement is in the correct order of radius size? a) Mn > Mn2+ > Cs b) Li+ > Li > Ra c) P < P3– < As3– d) Cr < Cr3+ < Ca e) Al3+ > Al > Si | Socratic
Periodic Trend: Atomic Radius Video Tutorial & Practice | Channels for Pearson+ Aug 24, 2023In decreasing order of atomic radius, the elements rank as follows: Tin (Sn), Germanium (Ge), Silicon (Si), and Carbon (C). Atomic radius generally increases as you move down a group and decreases as you move across a period on the periodic table. Explanation:
Atomic Radius Trend | Periodic Table | ChemTalk Periodic Trends in Atomic Size | CK-12 Foundation VIDEO ANSWER: It’s helpful to know the trend with a periodic table if you want to rank the elements in order of increasing atomic radius. The size of the nucleus is going to go down. We see a decrease in atomic radii.