Jul 27, 2023To calculate an atomic charge, simply subtract the number of electrons from the number of protons. How to Calculate Atomic Charge? The following two example problems outline how to calculate the Atomic Charge. Example Problem #1: First, determine the number of protons. In this example, the number of protons is given as 50.
The Electric Field due to a Half-Ring of Charge | by Rhett Allain | Geek Physics | Medium
Atoms have the same number of protons and neutrons, but when an atom gains electrons it becomes an anion. A nitrogen anion has 7 protons ans 10 electrons. Each proton has a charge of +1 and each electron has a charge of -1. What is the total charge of the atom?

Source Image: igcsetuition.blogspot.com
Download Image
Apr 29, 2023Atomic Charge Calculator How it works. The Atomic Charge Calculator is a simple, user-friendly tool that helps you quickly determine an atom’s atomic charge. You only need to input the number of protons and electrons, and the calculator will do the rest. It works by applying the atomic charge formula and displaying the result in electron
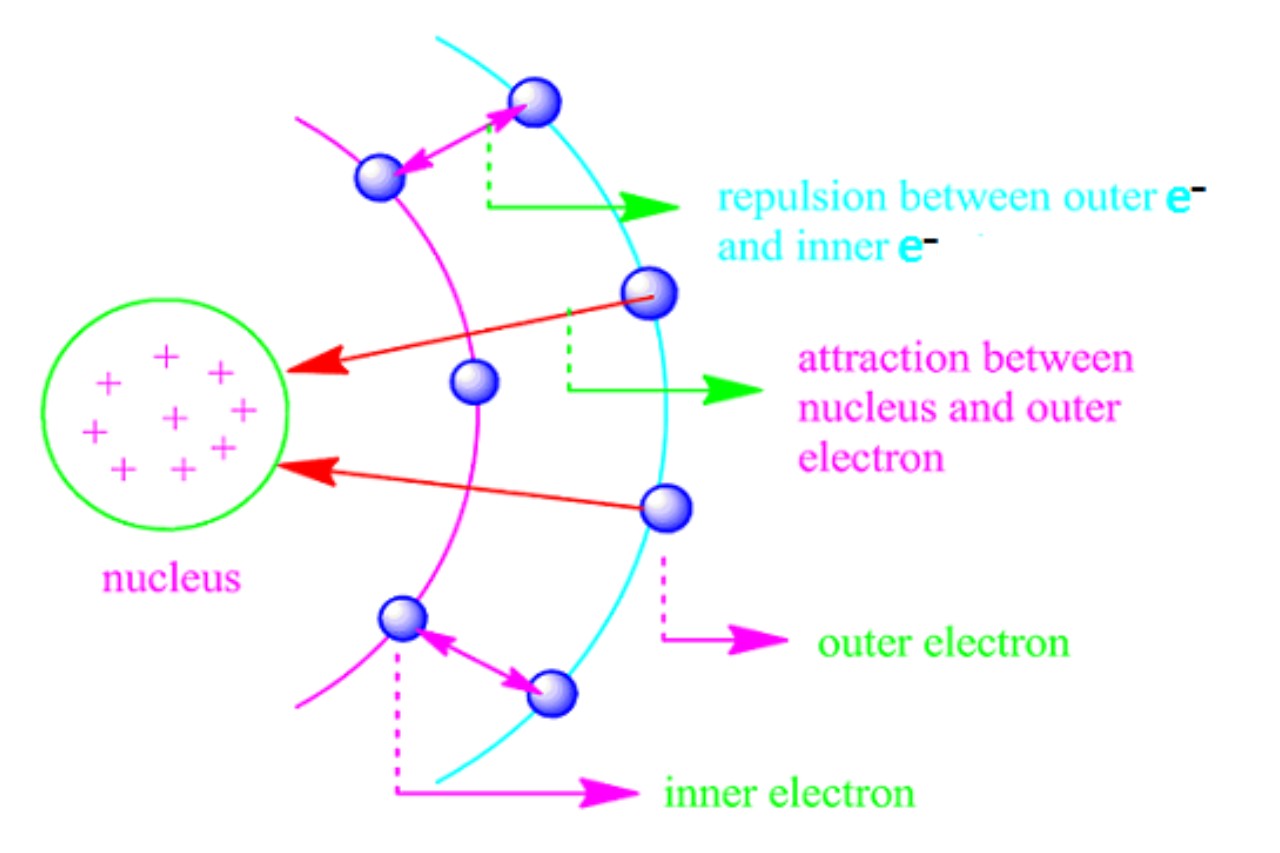
Source Image: facts.net
Download Image
Lindqvist — a blog about Linux and Science. Mostly.: 415. Briefly: making a polyhedral molecular figure in gdis If we know the charge density ρ in some volume of space, we can find the total charge by chopping up the volume into lots of small (actually infinitesimal) volumes , d τ, finding the charge on each , ρ ( r →) d τ, and adding up (integrating) the charges from each of the small volumes. tot vol. (10.3.1) (10.3.1) Q tot = ∫ vol. ρ ( r →

Source Image: study.com
Download Image
How Do You Find The Total Charge Of An Atom
If we know the charge density ρ in some volume of space, we can find the total charge by chopping up the volume into lots of small (actually infinitesimal) volumes , d τ, finding the charge on each , ρ ( r →) d τ, and adding up (integrating) the charges from each of the small volumes. tot vol. (10.3.1) (10.3.1) Q tot = ∫ vol. ρ ( r → Answer link The atom has an overall neutral charge This is when the number of electrons are equal to the number of protons. An ion (an atom that has gained or lost electrons) will have either a positive or a negative charge.
How to Count Protons & Electrons in Atomic Ions | Chemistry | Study.com
Step 1 : Determine the number of protons and the number of electrons in the arrangement. Step 2 : Subtract the number of electrons from the number of protons to get a number N. This represents the HOW TO DO THIS QUESTION PLEASE – brainly.com

Source Image: brainly.com
Download Image
Mass of Proton – Definition, Charge, Discovery, Properties with Videos Step 1 : Determine the number of protons and the number of electrons in the arrangement. Step 2 : Subtract the number of electrons from the number of protons to get a number N. This represents the

Source Image: byjus.com
Download Image
The Electric Field due to a Half-Ring of Charge | by Rhett Allain | Geek Physics | Medium Jul 27, 2023To calculate an atomic charge, simply subtract the number of electrons from the number of protons. How to Calculate Atomic Charge? The following two example problems outline how to calculate the Atomic Charge. Example Problem #1: First, determine the number of protons. In this example, the number of protons is given as 50.

Source Image: medium.com
Download Image
Lindqvist — a blog about Linux and Science. Mostly.: 415. Briefly: making a polyhedral molecular figure in gdis Apr 29, 2023Atomic Charge Calculator How it works. The Atomic Charge Calculator is a simple, user-friendly tool that helps you quickly determine an atom’s atomic charge. You only need to input the number of protons and electrons, and the calculator will do the rest. It works by applying the atomic charge formula and displaying the result in electron

Source Image: verahill.blogspot.com
Download Image
Element Charges Chart – How to Know the Charge of an Atom Jan 18, 2024Find the number of protons (atomic number). Find the number of neutrons by subtracting the number of protons from the mass number. Add the protons and neutrons. Omit the electrons, as their combined mass is very small compared to the mass of the nucleus. Congratulations! Now you have the atomic mass of that atom.

Source Image: sciencenotes.org
Download Image
Chemistry Net on X: “Lewis structure of SO2 – Simple Procedure for drawing Dot structures https://t.co/ZMu85kL3Y0 #chemistry #university #generalchemistry #alevels #biochemistry https://t.co/x3qURvYwdD” / X If we know the charge density ρ in some volume of space, we can find the total charge by chopping up the volume into lots of small (actually infinitesimal) volumes , d τ, finding the charge on each , ρ ( r →) d τ, and adding up (integrating) the charges from each of the small volumes. tot vol. (10.3.1) (10.3.1) Q tot = ∫ vol. ρ ( r →
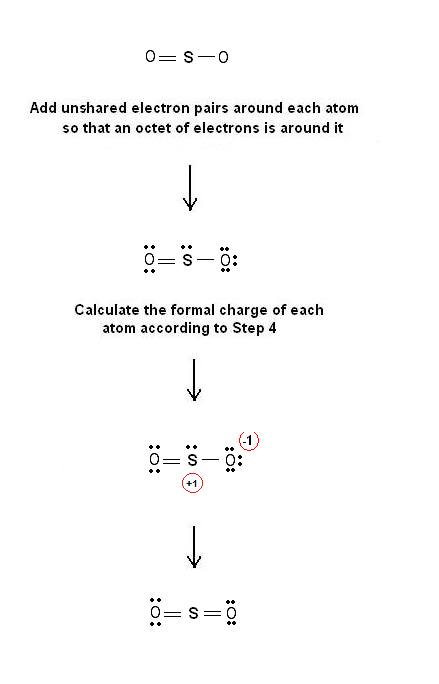
Source Image: twitter.com
Download Image
Chemistry Net on X: “Drawing Lewis Structures: An Easy Method https://t.co/XoxKb3uf2v https://t.co/smfibSXIXg #chemtwitter #chemistry #cheinformatics #Bioinformatics https://t.co/ptD2xAMqRp” / X Answer link The atom has an overall neutral charge This is when the number of electrons are equal to the number of protons. An ion (an atom that has gained or lost electrons) will have either a positive or a negative charge.

Source Image: twitter.com
Download Image
Mass of Proton – Definition, Charge, Discovery, Properties with Videos
Chemistry Net on X: “Drawing Lewis Structures: An Easy Method https://t.co/XoxKb3uf2v https://t.co/smfibSXIXg #chemtwitter #chemistry #cheinformatics #Bioinformatics https://t.co/ptD2xAMqRp” / X Atoms have the same number of protons and neutrons, but when an atom gains electrons it becomes an anion. A nitrogen anion has 7 protons ans 10 electrons. Each proton has a charge of +1 and each electron has a charge of -1. What is the total charge of the atom?
Lindqvist — a blog about Linux and Science. Mostly.: 415. Briefly: making a polyhedral molecular figure in gdis Chemistry Net on X: “Lewis structure of SO2 – Simple Procedure for drawing Dot structures https://t.co/ZMu85kL3Y0 #chemistry #university #generalchemistry #alevels #biochemistry https://t.co/x3qURvYwdD” / X Jan 18, 2024Find the number of protons (atomic number). Find the number of neutrons by subtracting the number of protons from the mass number. Add the protons and neutrons. Omit the electrons, as their combined mass is very small compared to the mass of the nucleus. Congratulations! Now you have the atomic mass of that atom.