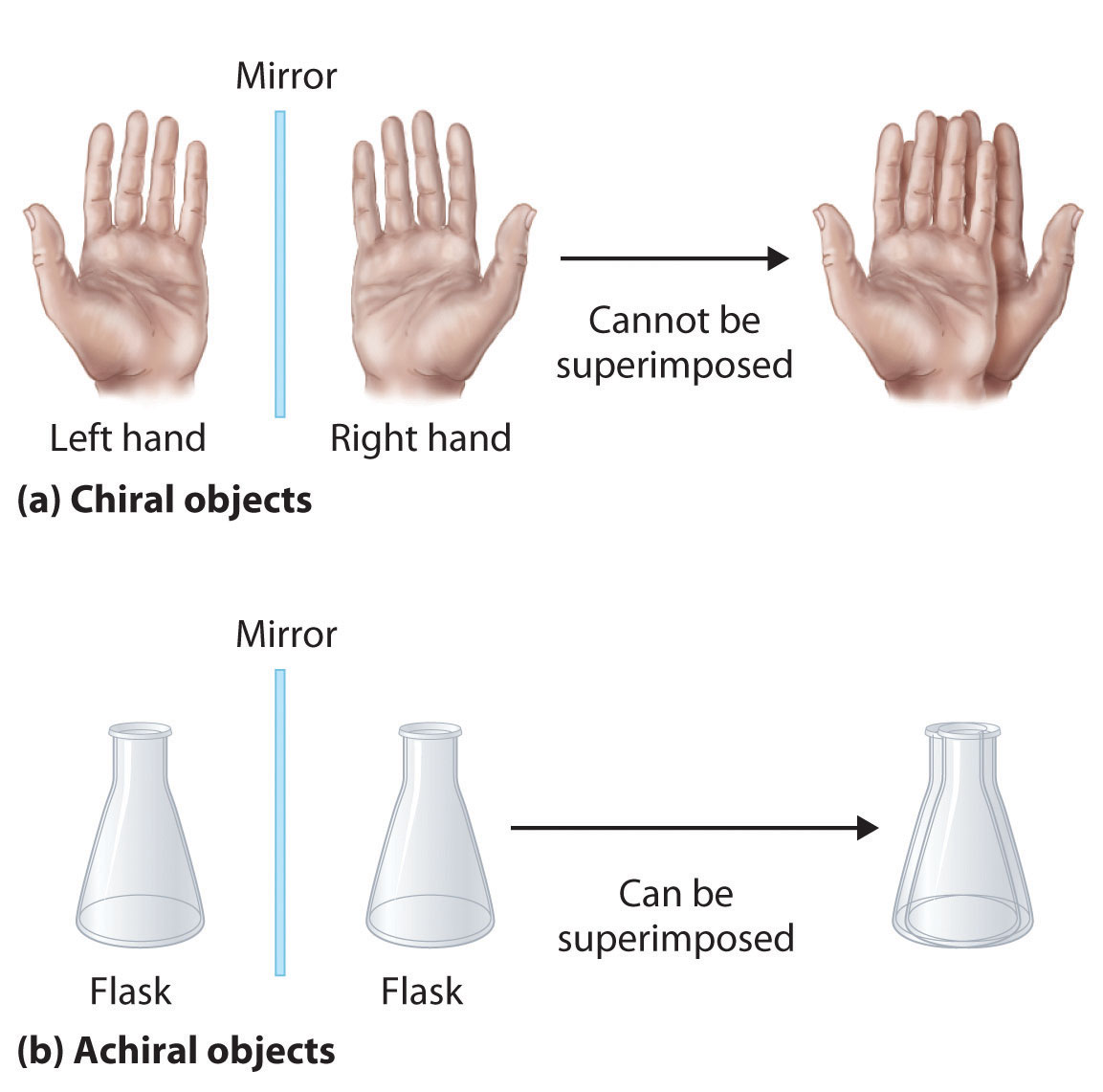
Chirality is an important geometric property relating to a molecule’s symmetry. A chiral molecule is non-superimposable with its mirror image, and has a “handedness” (think of shoes, which specifically go with a right or left foot). An achiral molecule is superimposable with its mirror image and do not have “handedness” (think of a baseball bat
Chapter 6: Chirality: Problems | PDF | Chirality (Chemistry) | Isomer
Explanation: H 2CBrF is achiral. H 2C = CH CF 3 is achiral. Br3C −C*H (Cl)Br has a chiral centre (starred), and could support a pair of optical isomers. H 3C − CH 2F is achiral. H 3CF is achiral. H 3C − *C(H)(Br)CH 2CH 3 is chiral. +P BrCl2(CH 3) is achiral. Answer link
Source Image: numerade.com
Download Image
Study with Quizlet and memorize flashcards containing terms like Select the statement that correctly differentiates between chiral and achiral molecules., Which of the following correctly describes a chirality center?, If the structure of a molecule is such that one half of the molecule is a mirror image of the other half, the molecule is said to have a _________of ________. and more.

Source Image: doubtnut.com
Download Image
Chiral and Achiral Molecules – Organic Chemistry | Socratic Look at your molecule, if you see any plane of symmetry the molecule must be achiral. If you don’t see a plane of symmetry that doesn’t mean the molecule is chiral. But checking for a plane is an easy first step and may often make the time consuming step of drawing and comparing structures unnecessary. – ron May 10, 2015 at 18:25

Source Image: homework.study.com
Download Image
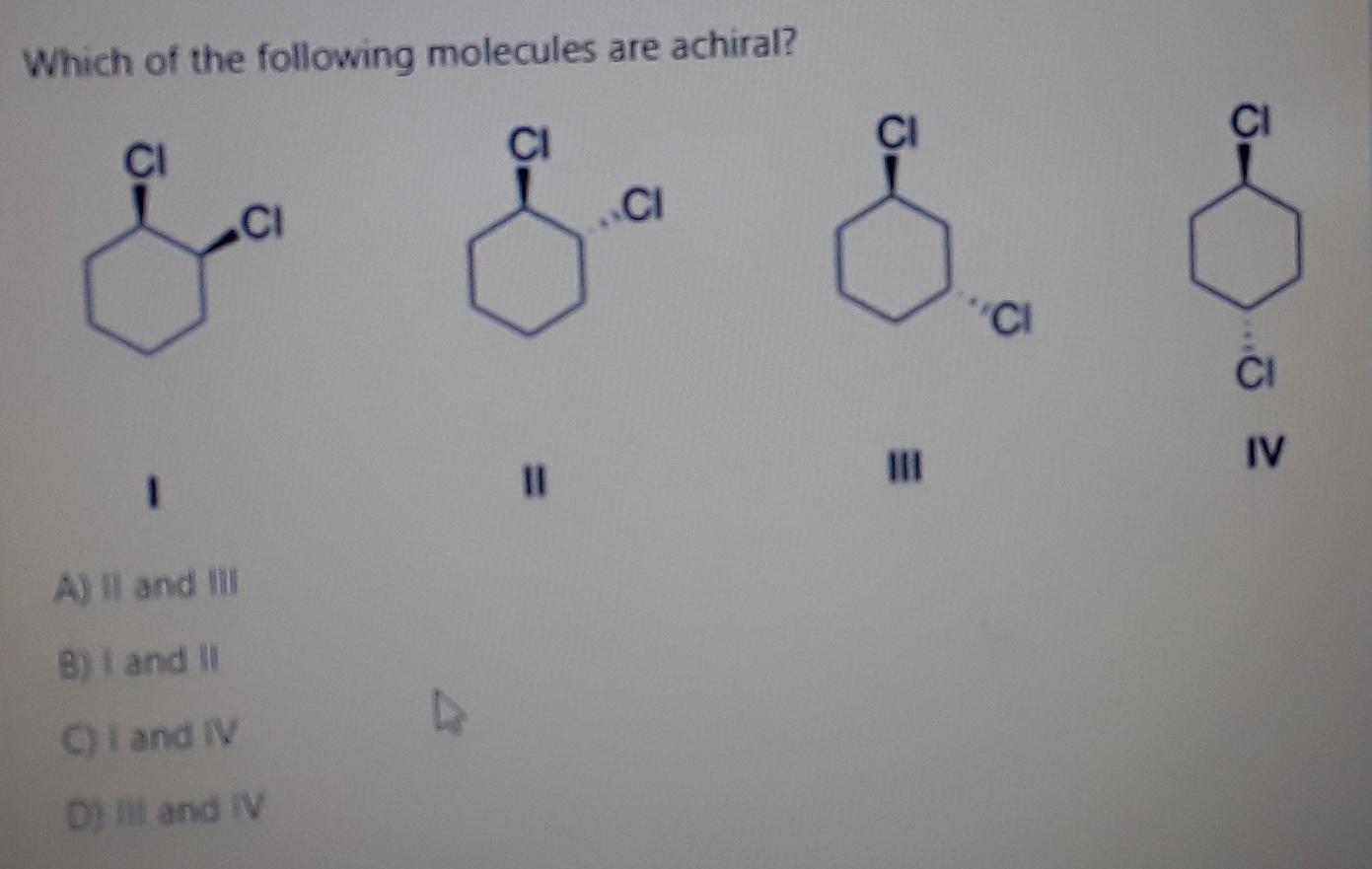
Which Of The Following Molecules Are Achiral
Look at your molecule, if you see any plane of symmetry the molecule must be achiral. If you don’t see a plane of symmetry that doesn’t mean the molecule is chiral. But checking for a plane is an easy first step and may often make the time consuming step of drawing and comparing structures unnecessary. – ron May 10, 2015 at 18:25 Apr 19, 2022Chirality is a symmetry property widespread in nature, and examples include the molecules of life (e.g., proteins, nucleic acids). A molecule is chiral when it cannot be superimposed on its mirror
Indicate if the following molecules are chiral molecules or achiral molecules. | Homework.Study.com
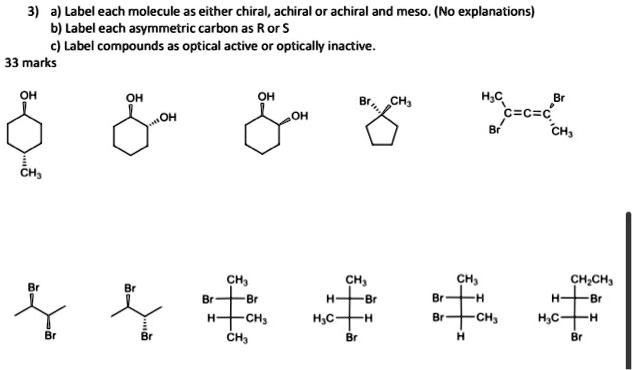
1) For the following compounds, star (*) each chiral center, if any. 2) Explain why the following compound is chiral. 3) Determine which of the following objects is chiral. a) A Glove. b) A nail. c) A pair of sunglasses. d) The written word “Chiral”. 4) Place an “*” by all of the chrial carbons in the following molecules. a) Erythrose, a four Solved Which of the following molecules are achiral? A) II | Chegg.com
Source Image: chegg.com
Download Image
Indicate if the following molecules are chiral molecules or achiral molecules. | Homework.Study.com 1) For the following compounds, star (*) each chiral center, if any. 2) Explain why the following compound is chiral. 3) Determine which of the following objects is chiral. a) A Glove. b) A nail. c) A pair of sunglasses. d) The written word “Chiral”. 4) Place an “*” by all of the chrial carbons in the following molecules. a) Erythrose, a four

Source Image: homework.study.com
Download Image
Chapter 6: Chirality: Problems | PDF | Chirality (Chemistry) | Isomer Chirality is an important geometric property relating to a molecule’s symmetry. A chiral molecule is non-superimposable with its mirror image, and has a “handedness” (think of shoes, which specifically go with a right or left foot). An achiral molecule is superimposable with its mirror image and do not have “handedness” (think of a baseball bat

Source Image: scribd.com
Download Image
Chiral and Achiral Molecules – Organic Chemistry | Socratic Study with Quizlet and memorize flashcards containing terms like Select the statement that correctly differentiates between chiral and achiral molecules., Which of the following correctly describes a chirality center?, If the structure of a molecule is such that one half of the molecule is a mirror image of the other half, the molecule is said to have a _________of ________. and more.
Source Image: socratic.org
Download Image
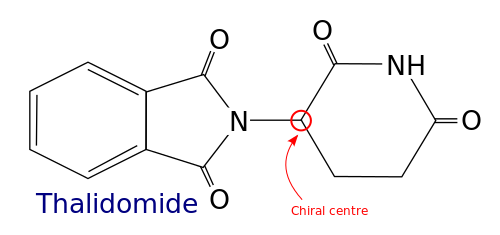
Difference Between Chiral and Achiral | Difference Between If an object is superimposable on its mirror image (for such case the object and its mirror image are exact identical), then this object is not chiral, that can be said as achiral. Figure 5.3d Cup is achiral Figure 5.3e Lego piece is achiral. In organic chemistry, we are interested in organic molecules that are chiral.
Source Image: differencebetween.net
Download Image
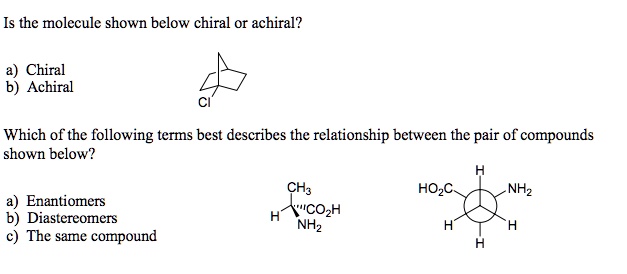
SOLVED: Is the molecule shown below chiral or achiral? a) Chiral Achiral Which of the following terms best describes the relationship between the pair of compounds shown below? CH3CO2H NH2 HOCH2 NH2 Look at your molecule, if you see any plane of symmetry the molecule must be achiral. If you don’t see a plane of symmetry that doesn’t mean the molecule is chiral. But checking for a plane is an easy first step and may often make the time consuming step of drawing and comparing structures unnecessary. – ron May 10, 2015 at 18:25
Source Image: numerade.com
Download Image
Identify chiral and achiral molecules in each of the following pair of compounds – YouTube Apr 19, 2022Chirality is a symmetry property widespread in nature, and examples include the molecules of life (e.g., proteins, nucleic acids). A molecule is chiral when it cannot be superimposed on its mirror

Source Image: m.youtube.com
Download Image
Indicate if the following molecules are chiral molecules or achiral molecules. | Homework.Study.com
Identify chiral and achiral molecules in each of the following pair of compounds – YouTube Explanation: H 2CBrF is achiral. H 2C = CH CF 3 is achiral. Br3C −C*H (Cl)Br has a chiral centre (starred), and could support a pair of optical isomers. H 3C − CH 2F is achiral. H 3CF is achiral. H 3C − *C(H)(Br)CH 2CH 3 is chiral. +P BrCl2(CH 3) is achiral. Answer link
Chiral and Achiral Molecules – Organic Chemistry | Socratic SOLVED: Is the molecule shown below chiral or achiral? a) Chiral Achiral Which of the following terms best describes the relationship between the pair of compounds shown below? CH3CO2H NH2 HOCH2 NH2 If an object is superimposable on its mirror image (for such case the object and its mirror image are exact identical), then this object is not chiral, that can be said as achiral. Figure 5.3d Cup is achiral Figure 5.3e Lego piece is achiral. In organic chemistry, we are interested in organic molecules that are chiral.